how to draw molecular orbital diagram for heteronuclear molecules
Depending on if it is a homonuclear case where the bonding atoms are the same or a heteronuclear case where the bonding atoms are. Drawing molecular orbital diagrams is one of the trickier concepts in chemistry.
Molecular Orbitals Molecular Orbital Theory Sparknotes
In case of beryllium hydride there are 3 atoms overlapping simultaneously.

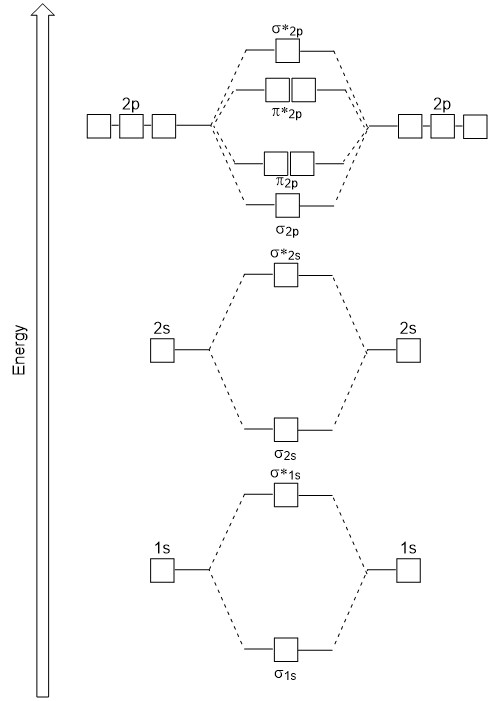
. Lines often dashed diagonal lines connect MO levels with their constituent AO levels. Taking the internuclear axis as the z-axis we have. Now we have two of the same atomic orbital diagrams laid out.
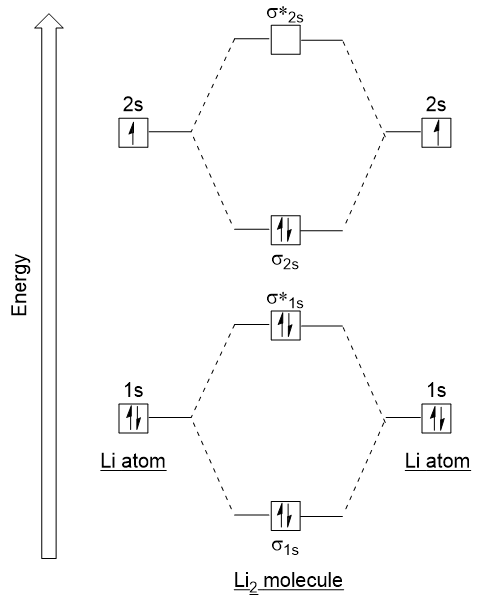
Draw the MO diagram for B_2. Draw out the MO diagram and label in the valence electrons. Now MO diagrams are only simple for elements of the second row of the periodic table ceLi through ceNe.
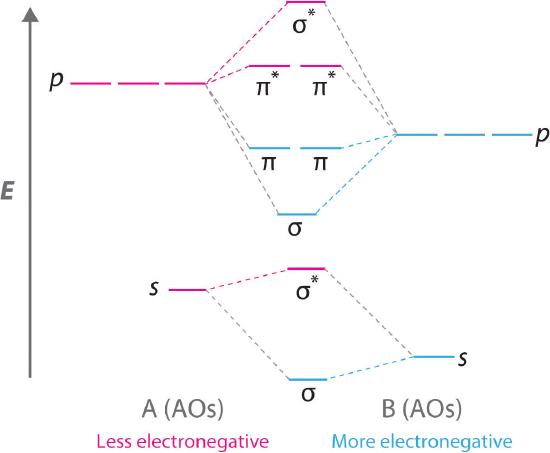
Boron has 2 electrons in the 2s orbitals and 1 electron in the 2p orbital. Mulliken in 1932 to mean. The molecular orbitals in the heteronuclear case will in general be concentrated more around one nucleus than the other.
Begingroup Mulikens 1935 Electronic Structures of Polyatomic Molecules. Then for the molecular orbital diagram we examine how these atomic orbitals interact with each other in a linear combination of atomic orbitals LCAO. Thus the rule becomes.
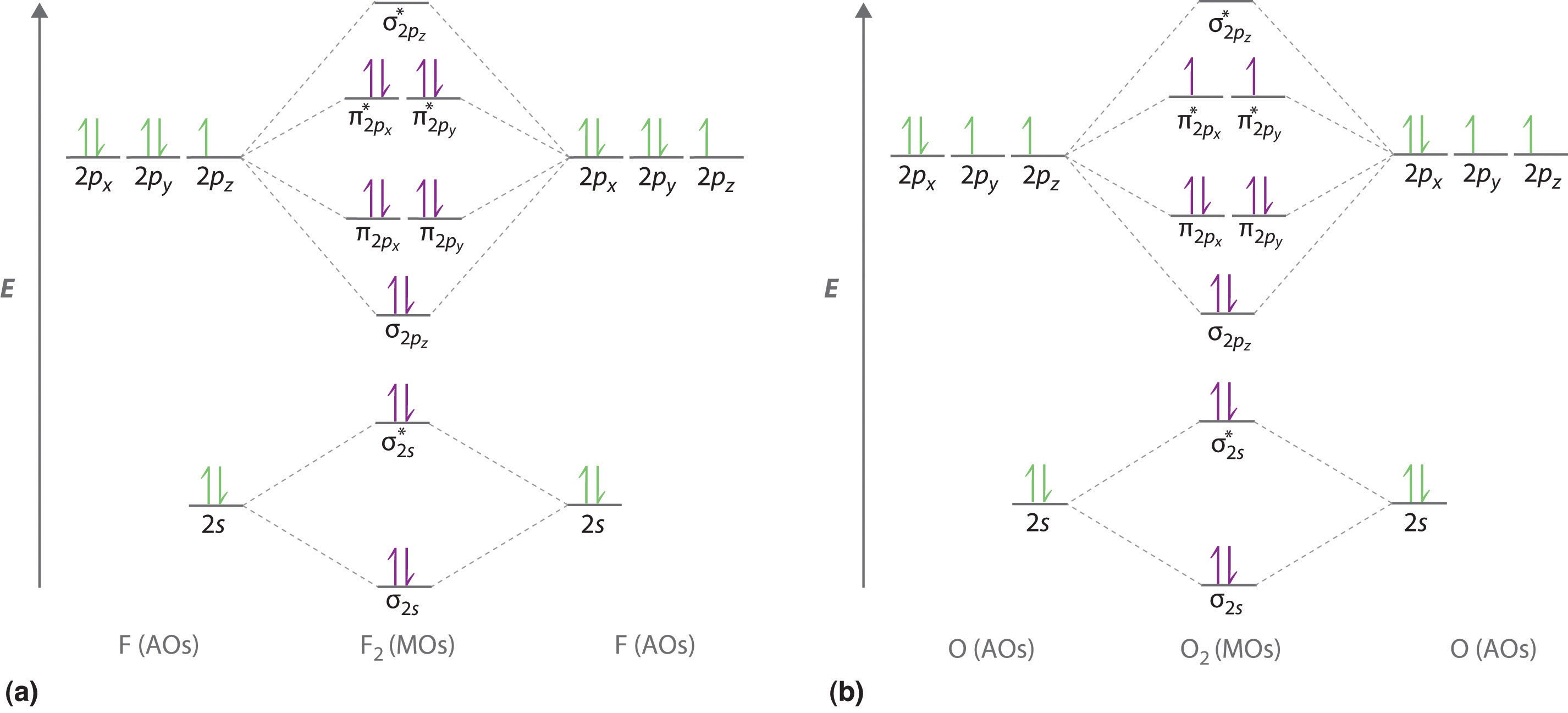
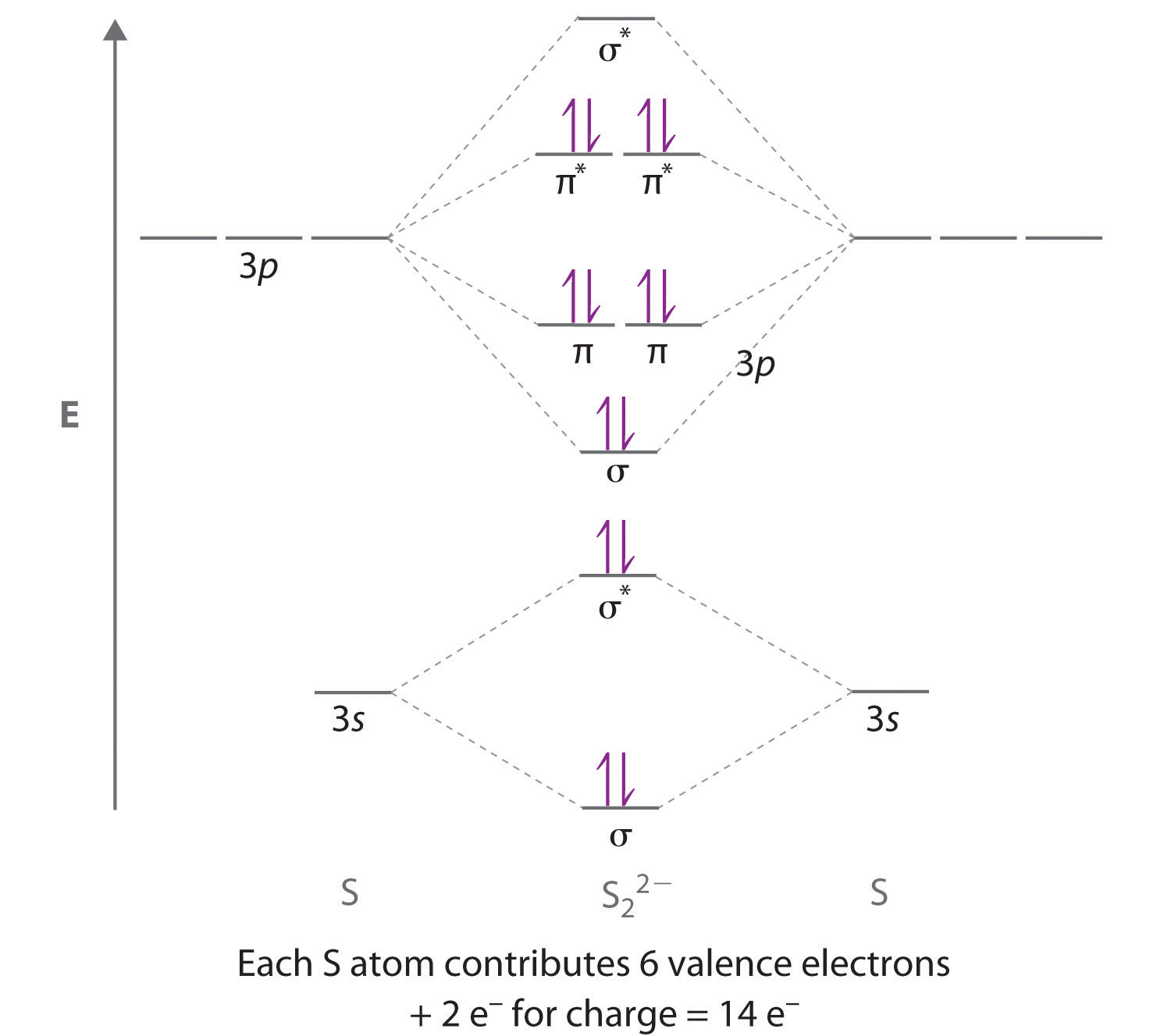
LCAO of Heteronuclear Diatomics Answers 110 KB. Molecular orbital diagrams are diagrams of molecular orbital MO energy levels shown as short horizontal lines in the center flanked by constituent atomic orbital AO energy levels for comparison with the energy levels increasing from the bottom to the top. The F 2s is nonbonding.
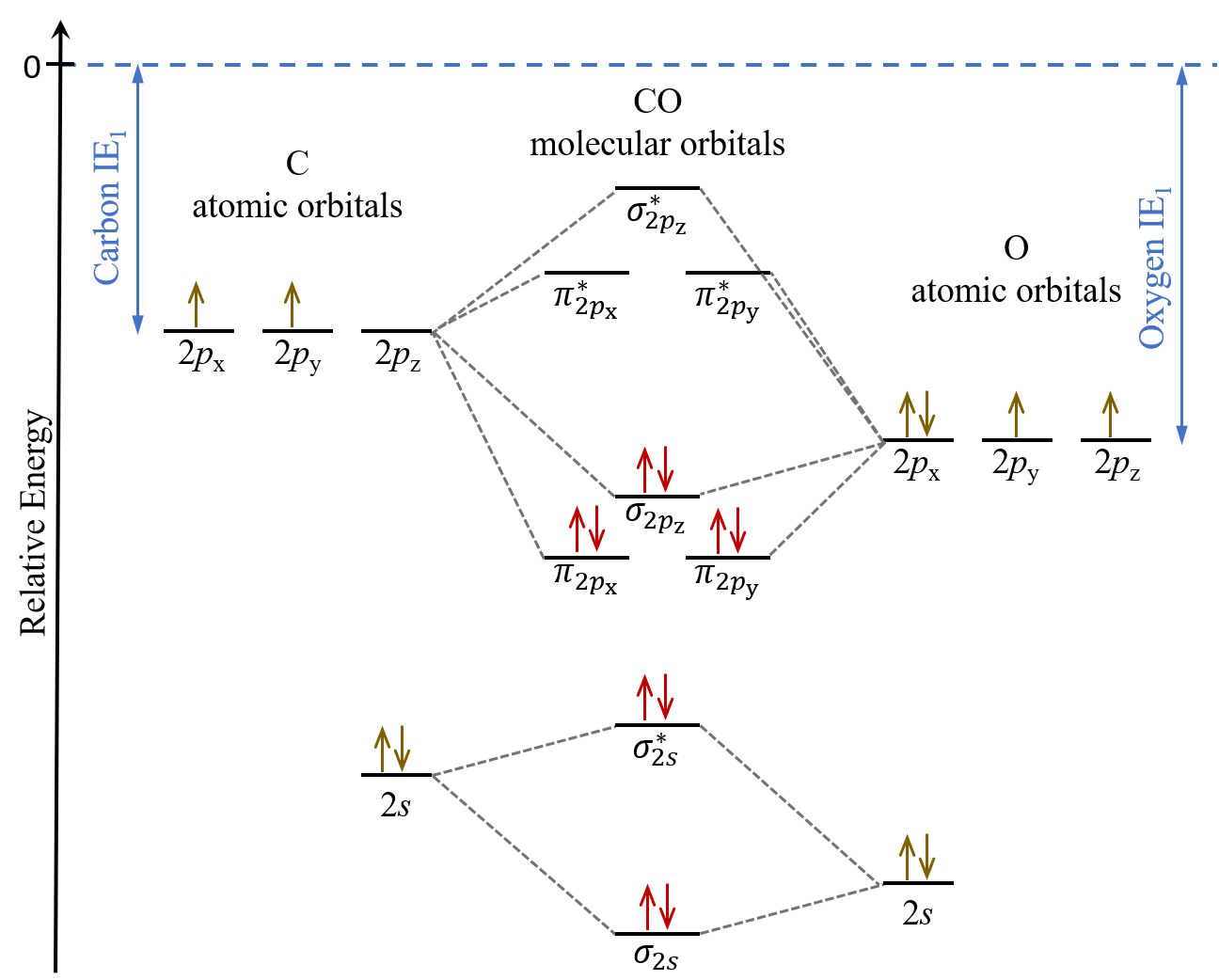
Construct the Molecular Orbital Diagram for carbon monoxide CO. The further to the right your element is the lower its energy levels are. Now I know that the more electronegative atoms orbitals are going to be lower in energy than the less electronegative atom.
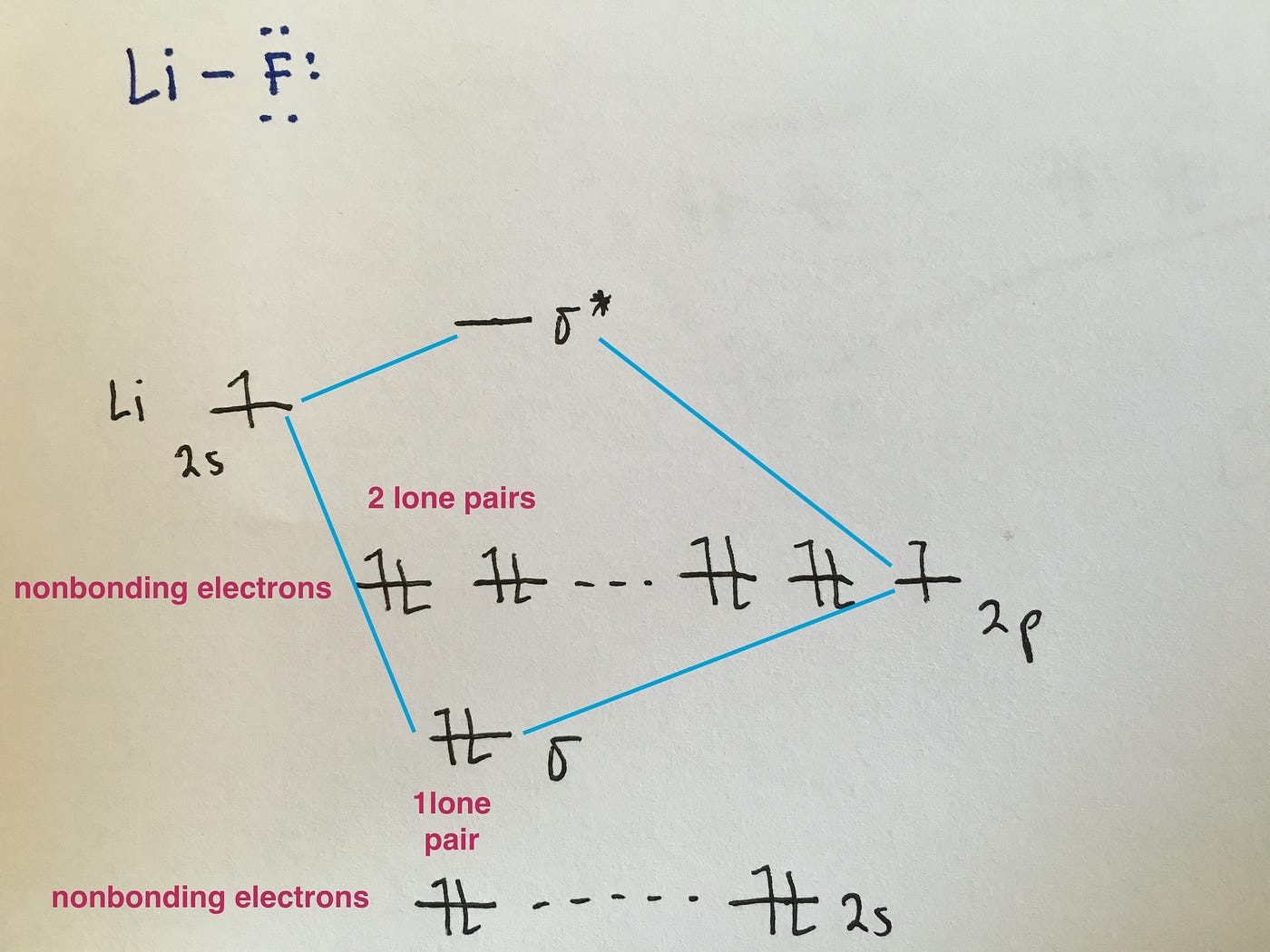
Molecular Orbital Diagrams of Heteronuclear Diatomics Answers 173 KB. Summary MO Theory LCAO-MO Theory is a simple method for predicting the approximate electronic structure of molecules. HF nb σ σ Energy H 136 eV 1s F 186 eV 402 eV 2s 2p So HF has one σ bond and three lone electron pairs on fluorine.
Find the valence electron configuration of each atom in the molecule. In polyatomic molecules we can have more than two atoms combining eg. Decide if the molecule is homonuclear of heteronuclear.
LCAO of Heteronuclear Diatomics. I have learned so far that there are two ways to set up MO diagrams for homo-nuclear diatomic molecules. Contour maps of the molecular orbital charge densities for H 2 O.
The terms atomic orbital and molecular orbital were introduced by Robert S. Thats it for the MO diagram of B_2. FUNDAMENTAL STEPS IN DERIVING MO DIAGRAMS.
The molecular orbitals which describe the motion of a single electron in a molecule containing two unequal nuclear charges will not exhibit the g and u symmetry properties of the homonuclear diatomic case. One was with the pi 2p bonding MO below the σ2p bonding orbital and the reverse for atoms with an atomic number 8. Reference to the forms of the charge density contours for the la molecular orbital substantiates the above remarks regarding the properties of this orbital.
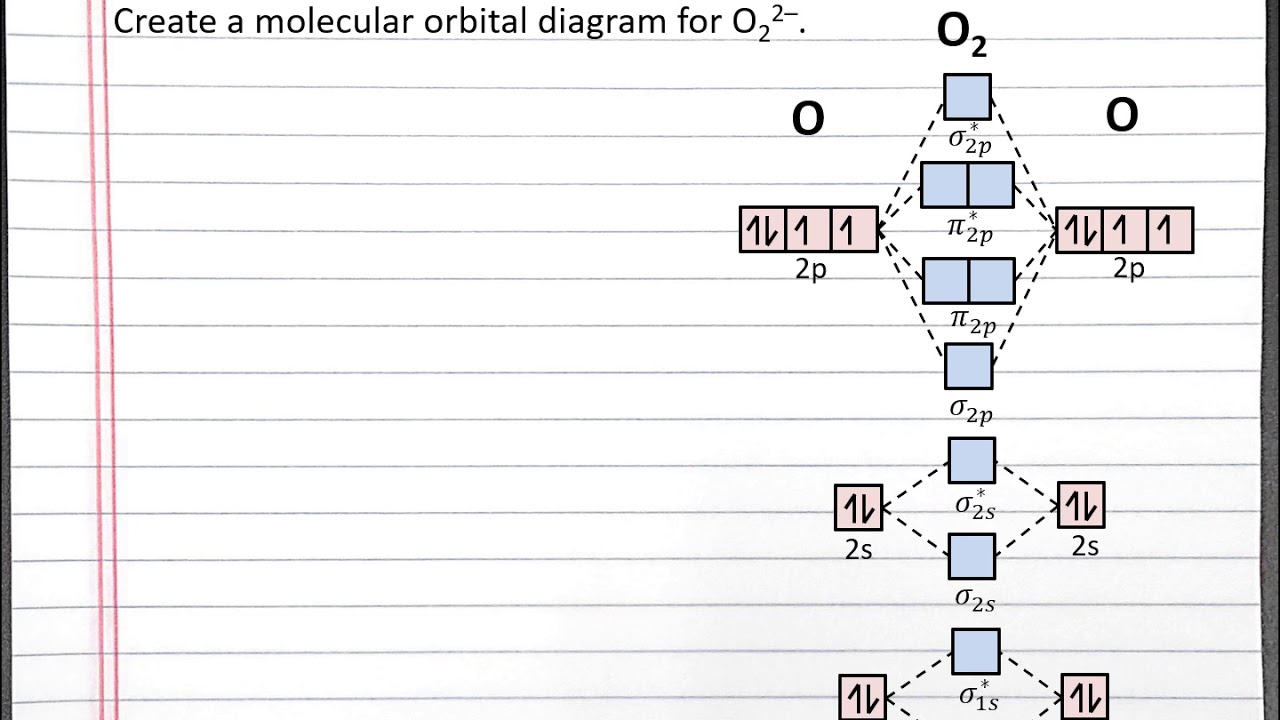
Molecular Orbital Diagrams of Heteronuclear Diatomics. Heres how this goes of course the ns are compatible with the ns. Draw the molecular orbital diagram for the oxygen molecule O2.
Ammonia and Water Type Molecules and Their Derivatives givens energy values for water MOs with the two non-bonding lone pair orbitals being 32. In this case were using the standard one. In chemistry a molecular orbital is a mathematical function describing the location and wave-like behavior of an electron in a moleculeThis function can be used to calculate chemical and physical properties such as the probability of finding an electron in any specific region.
First step is to determine which MO diagram were using. Molecular Orbital Diagrams of Heteronuclear Diatomics. Molecular Orbitals for Heteronuclear Molecules.
Hydrogen Helium 1s -100 Ry -181 Ry a Use the energy values in the table above to help you develop a valencemolecular orbital energy level diagram for the helium protonate ion 𝐻𝐻𝐻𝐻𝐻𝐻. Next well see that symmetry will help us treat larger. Abstract TLDR Molecular orbital diagrams are a fantastic way of visualizing how molecular orbitals form using what we already understand about sigma and pi bonds.
Bond order of molecules can be calculated from the equation 12Nb-Na where Nb is the number of. Energy Values for the Atomic Orbitals of Hydrogen and Helium Atoms. Such as H 2O NH 3 and CH 4 However notice the difference between orbitals of homonuclear diatomic for.
Involving heavier atoms makes it harder to guess at molecular orbital diagrams and there is need for quantum chemistry calculations. MO Diagram for HF The AO energies suggest that the 1s orbital of hydrogen interacts mostly with a 2p orbital of fluorine. How to draw molecular orbital diagram for co2.
8 - Drawing Molecular Orbital Diagrams. From this diagram calculate the From this diagram calculate the A. Fill molecular orbitals using energy and bonding properties of the overlapping atomic orbitals.
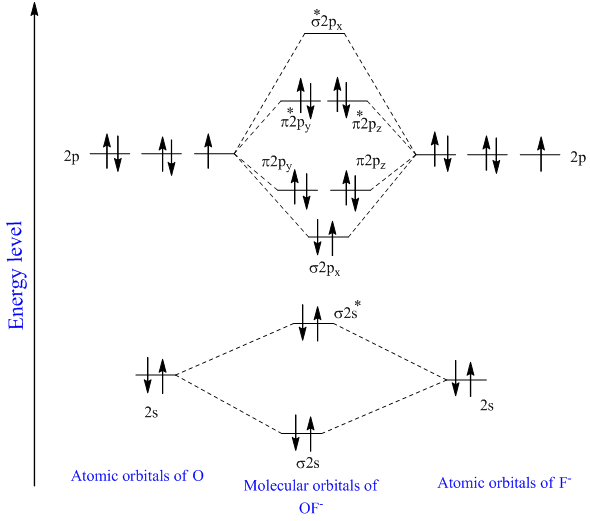
Heteronuclear Diatomic Molecules. The maps for the la 1 2a 1 3a 1 and 1b 2 orbitals all doubly-occupied are shown in the plane of the nuclei. Heteronuclear Diatomic Molecules are composed of 2 different elements bonded together.
In carbon monoxide CO the oxygen 2s orbital is much lower in energy than the carbon 2s orbital so the degree of mixing is low. In heteronuclear diatomic molecules atomic orbitals only mix when the electronegativity values are similar. The g and u subscripts no longer apply because the molecule lacks a center of symmetry.
Molecular Orbital Diagrams of Heteronuclear Diatomics. An anti-bonding orbital is written as the bond with the star superscripted onto it. Use the diagram to predict properties of the molecule.
Draw the orbital diagram for ion Co 2. 8 Drawing Molecular Orbital Diagrams Flux Science Molecular orbitals in Carbon Monoxide CO. Find the valence electron of each atom in the CN molecule.
High-spin octahedral d7 has LFSE o. Building Molecular Orbital Diagrams for Homonuclear and Heteronuclear Diatomic Molecules Due to symmetry of the molecule homonuclear MOs are less difficult to derive than heteronuclear molecules and polyatomic molecules.

8 Drawing Molecular Orbital Diagrams Flux Science
2 6 Molecular Orbital Theory Chemistry Libretexts
8 Drawing Molecular Orbital Diagrams Flux Science

Question 13 Molecular Orbitals For Heteronuclear Diatomic Molecules Using The Above Molecular Orbital Diagram For Co Homeworklib
Molecular Orbital Diagrams Simplified By Megan A Lim Medium
Molecular Orbitals Introductory Chemistry
Molecular Orbitals Introductory Chemistry 1st Canadian Edition
2 6 Molecular Orbital Theory Chemistry Libretexts
Delocalized Bonding And Molecular Orbitals

Molecular Orbital Diagram Of Polyatomic Co2 Molecules Chemical Bonding Molecular Structures Youtube

Solved Draw A Molecular Orbital Diagram For Each Of The Chegg Com
Chem 101 Creating A Molecular Orbital Diagram For A Diatomic Ion In The Second Row With Aleks Youtube

8 5 Molecular Orbital Theory Chemistry
D6 5 Mos For Heteronuclear Diatomic Molecules Chemistry 109 Fall 2021

Mo Diagram Of Hydrogen Helium Carbon Molecule Youtube

Molecular Orbital Diagrams Simplified By Megan A Lim Medium
Solved Chapter 5 Problem 10p Solution Inorganic Chemistry 5th Edition Chegg Com

Molecular Orbital Theory Heteronuclear Diatomic Cyanide Cn Example Youtube